Zinc and cadmium complexes with optical properties
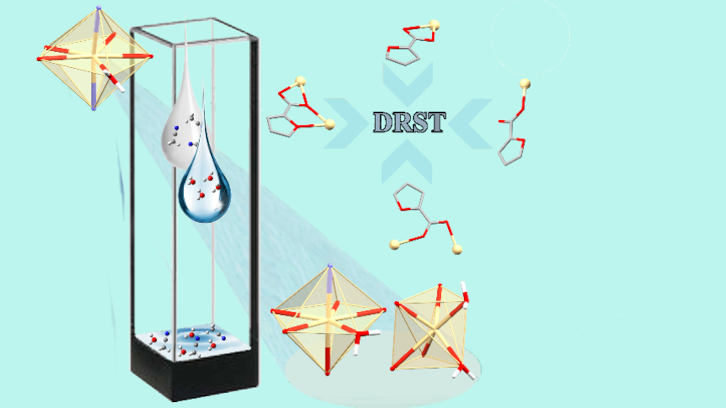
Crystal engineering plays an essential role in understanding how organic ligands and metal ions are assembled when synthesizing materials with special properties. Researchers from the Department of Chemistry have synthesized four zinc and cadmium compounds with 2-furoic acid, including a coordination polymer, and have studied their optical properties. The study expands the understanding of coordination polymers and the role of solvents in their formation.
Understanding the factors governing the self-assembly of organic ligands with metal ions is essential to engineering target molecular arrangements with the desired properties. Small modifications of the synthetic conditions lead to the obtention from monomers to coordination polymers (CPs).
The d10 coordination complexes has emerged as an outstanding research area benefitting from their application on the field optical materials (luminophores, sensing devices, photoelectrical conductivity). The Zn(II) and Cd(II) ions enables a great structural diversity and coordination versatility. For Zn(II), the coordination number present a range from four to six, and Cd(II) the coordination number up to seven. Zn(II) and Cd(II) carboxylates display dimeric arrays. After of the introduction of pyridine derivatives, the preferred arrangements are barely altered, only leading to a further increase in dimensionality.
Based on the potential coordination of 2-furoic acid (2-FA), we prepared five Zn(II) and Cd(II) complexes to study the behavior of the O-atom from furane and the competitiveness of the M-O bond formation in methanol.
In this work, we assayed the reaction between M(OAc)2·2H2O (M = Zn(II), Cd(II); OAc = acetate), 2-FA and 4-acetylpyridine (4-Acpy) or isonicatinamide (Isn) in methanol, obtaining monomeric and dimeric complexes. The coordination numbers of the four compounds are between five and seven. The recrystallization of the monomer compound ([Cd(2-FA)2(4-Acpy)2(OH2)]) in acetonitrile leading to an intricate coordination polymer (CP). In this polymer the O-furane coordinate to Cd. The process resulted in a dissolution-recrystallization structural transformation (DRST).
Photophysical properties in solution were analyzed and their quantum yields calculated.
Interestingly, the competitiveness between water, carboxylate, and a coordinating O-atom from a furane ring has been demonstrated by tracing the formation of CP by a DRST process through X-ray data and fluorescence experiments.
This paper, contributed to the limited number of cases in which DRST processes evidence the crucial role of the solvents in controlling the engagement of weakly coordination atoms, in particular of O-furane atoms.
Departament of Chemistry
Universitat Autònoma de Barcelona
References
Daniel Ejarque, Francisco Sánchez-Férez, Núria Félez-Guerrero, Teresa Calvet, Mercè Font-Bardiac i Josefina Pons. Pyridine-driven assembly of Zn(II) and Cd(II) complexes with 2-furoic acid. The role of water in a structural transformation. CrystEngComm. 2023, 25, 2739-2754. DOI: /10.1039/D3CE00104K

