Synthesis and characterization of co-crystal, Cu(II) monomer and Cu(II) polymer
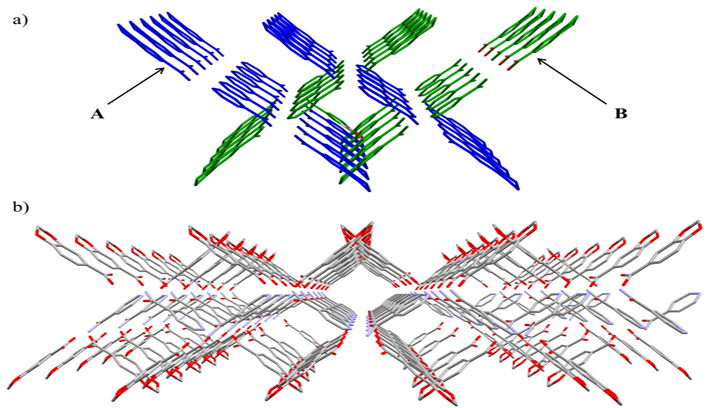
In terms of the design of new organic and inorganic materials, those establishing intermolecular forces are of great interest for their utility as biological models and in materials science. One of these intermolecular forces are hydrogen bonds, whose characteristics are useful in crystal engineering.
The carboxylate acids, formed partly by a carboxylate group, can form strong hydrogen bonds (O-H) when in presence of other molecules and organic compounds that have N-H groups (amines, amides or pyridines) in their structure. On the one hand, when the hydrogen bonds are formed directly between the O-H of the carboxylate group and the N-H group without the presence of metals, cocrystals are formed. The importance of cocrystals lies in the possibility of modifying the physical properties such as melting point, solubility, and conductivity, among other aspects.
On the other hand, during the formation of these compounds (co-crystals), if there is a presence of a metal ion such as Cu(II), the competition between the formation of cocrystals and complexes is observed. This is the process described in this paper that, in particular, presents the three compounds: cocrystal, Cu(II) monomer and Cu(II) polymer. Moreover, in this case, the carboxylic acid is piperonylic acid (HPip) and the amine is isonicotinamide (Isn).
In a synthesis of the research described in this paper, what is observed is the competition between the formation of a cocrystal and a Cu(II) monomer, and in another synthesis, the competition between the formation of a monomer and a polymer. In this paper, it has been possible to obtain the cocrystal and monomer separately, but the synthetic method for obtaining only the polymer was not discovered. Both compounds simultaneously crystallized in a 1.5:1 proportion, monomer (violet) and polymer (green).
All compounds were characterized by an X-ray crystal structure, which has allowed for the analysis and study of the molecular and supramolecular structures, as well as for elemental analyses and FTIR-ATR spectroscopy. Moreover, the cocrystal has been characterized by 1H and 13C NMR spectroscopies, while the monomer and polymer also were characterized by UV-Vis spectroscopy.
Finally, Hirshfeld surface analysis, energy frameworks and lattice energy calculation were performed for the three compounds. Hirshfeld analysis pinpointed the role of isonicotinamide. Furthermore, energy framework calculations distinguished the isonicotinamide as the driving cohesive force, while piperonylic acid itself mainly participates in the London dispersion forces. Lattice energy calculation showed a notable value as a consequence of the larger number of interactions promoted by the isonicotinamide ligand.
References
“Isonicotinamide-Based Compounds: From Cocrystal to Polymer” Francisco Sánchez-Férez, Daniel Ejarque, Teresa Calvet, Mercè Font-Bardía, Josefina Pons, Molecules, 2019,24,4169
1) B.-H. Ye, M.-L. Tong, X.-M. Chen, Coord. Chem. Rev. 2005, 249, 545-565.
2) A.R. Choudhury, G.R. Desiraju, A.G. Dikundwar, R. Dubey, N. Duggirala, P.P. Ghogale, S. Ghosh, P.K. Goswami, N.R. Goud, R.R.K.R. Jetti, Cryst. Growth Des. 2012, 12, 2147-2152.
3) F. Sánchez-Férez, M. Guerrero, J.A. Ayllón, T. Calvet, M. Font-Bardía, J. Giner Planas, J. Pons, Inorg. Chim. Acta 2019, 487, 295-306.
4) J. Soldevila-Sanmartín, J.A. Ayllón, T. Calvet, M. Font-Bardía, C. Domingo, J. Pons, Inorg. Chem. Commun. 2016, 71, 90-93.
5) A. Mukherjee, Cryst. Grwth Des. 2015, 15, 3076-3085.

