New copper compounds synthesized and characterized
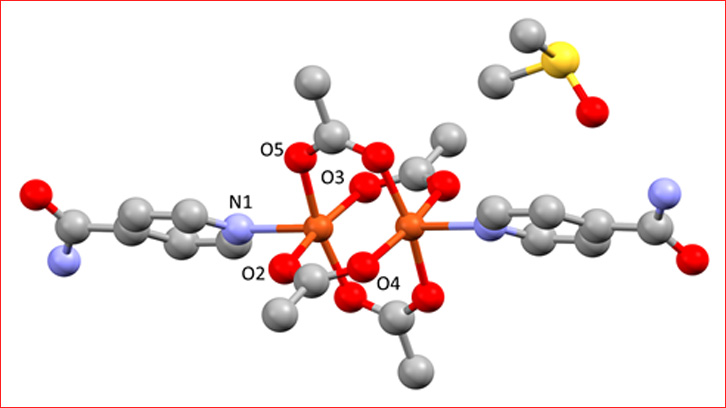
The study of the coordination chemistry of Cu(II) complexes with carboxylate groups has a great interest due to their labile nature and versatility. In particular, the acetate group provides the primary basic structure motif for constructing coordination complexes. Cu(II) acetates have been widely used not only as a starting reagent but also in the synthesis of coordination compounds and present different structures.
The most common array when Cu(II) acetates are in presence of monodentate N-donor ligands.
The different structural compounds and coordination modes of the acetate ligand is governed for different factors as solvent, pH, temperature or concentration.
Numerous dimeric Cu(II) compounds [CuX2L2L]2 (X = acetate or derivatives; L = pyridine or derivatives). One auxiliary ligand is the isonicotinamide (Isn), which is antitubercular, antipyretic, fibrinolytic, and antibacterial medicinal agent. It is worthwhile to mention that Isn has a higher pKa value of 10.61. In addition, it is a valuable directing motif for its role as a main stone in the resulting architecture. This is due to its characteristic amide-amide patters and its potential for constructing supramolecular 1D chains.
In the field of supramolecular chemistry, suitable directing motifs, either belonging to N-aromatic donor or carboxylic ligands have been thoroughly studied.
In this study, we present the reactivity of the compound [Cu(µ-OAc)(µ-Pip)(MeOH)]2 (OAc = acetate anion, Pip = carboxylic acid, MeOH = methanol) with Isn ligand in different solvents (methanol, N,N-dimethylformamide, water, dimethylsulfoxide and acetonitrile) with the finality to study the solvent-dependent formation of the set compounds (monomers and dimers).
In this paper, we have synthesized and characterized five compounds: The monomer is obtaining where the solvent is acetonitrile, and with the other solvents the compounds obtaining are dimers.
All compounds have been characterized by analytical and spectroscopic techniques, thermogravimetric analysis, and for all compounds the X-ray crystal structures have been resolved. In addition, we analyzed and studied the supramolecular structures of all compounds, obtained 2D and 3D networks.
References
"Solvent dependent formation of Cu(II) complexes based on isonicotinamide ligand" Francisco Sánchez-Férez, Laura Bayés, Mercè Font-Bardía, Josefina Pons, Inorganica Chimica Acta, 2019, 494, 112-122. https://doi.org/10.1016/j.ica.2019.05.010
F.P.W. Agterberg, H.A.J. Kluit, W.L. Driessen, H. Oevering, W. Buijs, M.T. Lakin, A.I. Spek, J. Reedijk, Inorg. Chem. 1997, 36, 4321-4328.
M. Swadzba-Kwasny, L. Chancelier, S. Ng, H.G. Manyar, C. Hardacre, P.Nockermann, Dalton Trans. 2012, 41, 219-227.
M. Arici, O.Z. Yesilel, E.Acar, N. Dege, Polyhedron 2017, 127, 293-301.
F. Sánchez-Férez, M. Guerrero, J.A. Ayllón, T. Calvet, M. Font-Bardía, J.G. Planas, J. Pons, Inorg. Chim. Acta, (2019) 487, 150-157.
J. Soldevila-Sanmartín, J.A. Ayllón, T. Calvet, M. Font-Bardía,C. Domingo, J. Pons, Inorg. Chem. Commun. 2016; 71, 90-93.

