Hybrid Protein Granules for the Biotechnology of the Future
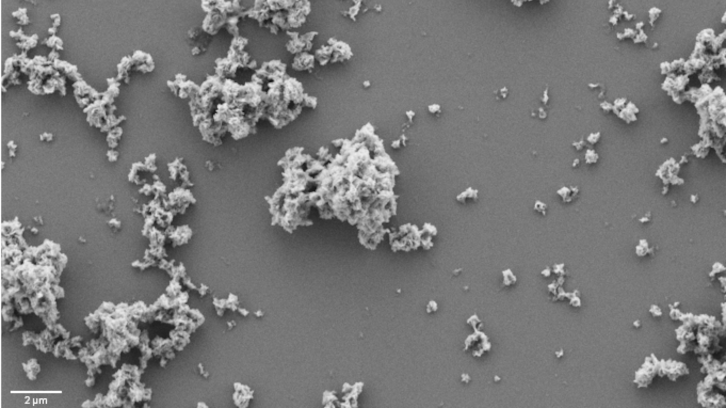
Proteins can form more complex structures to be stored and released when needed. The research group in Nanobiotecnology at UAB has developed a new concept of artificial protein granules capable of acting as protein depots and libering them over time. This breakthrough could inspire multiple aplications with huge potential, such as the combination of drugs and vaccines.
Proteins play key roles in most biological processes. In some cases, proteins organize into more complex structures to be stored and released in a controlled manner when needed. In nature, amyloid structures function as dynamic systems for protein storage and release, used by organisms as diverse as bacteria and humans, who use them, for example, in the function of hormones in our endocrine system.
Inspired by this concept, our research group in Nanobiotecnology has developed a new concept of artificial protein granules capable of acting as protein depots with potential biomedical and biotechnological applications, releasing them in a sustained manner over time. These granules are generated in a controlled way from recombinant proteins, which are produced by microorganisms or other cells using their natural ability to synthesize these biomolecules.
Unlike natural amyloids, we have demonstrated that this new system does not require the proteins to share a similar structure to be able to interact and organize together into hybrid granules. Thus, we have succeeded in combining in a unique protein-based material different proteins as different as an active enzyme and a fluorescent protein, by incorporating into each sequence a small histidine-rich region, a key amino acid with the ability to interact with divalent cations such as zinc. This histidine-zinc interaction enables the formation of stable hybrid granules that preserve the function of the original proteins, overcoming existing limitations in biomimetic amyloid structures.
This breakthrough allows us to envision multifunctional protein depots with a potential significant impact in biomedical and biotechnological applications, such as combining drugs or vaccines into a single, more potent entity, or creating enzymatic catalysts that can trigger multiple reactions in sequence. Thanks to this discovery, we have taken a step forward in creating more versatile and efficient artificial protein depots, bringing us closer to generating new biomimetic materials with high applied potential.
This research, published in the journal International Journal of Biological Macromolecules, would not have been possible without the multidisciplinary collaboration between various researchers and institutions, as well as the financial support of scientific-technical projects, particularly project PID2022-1368450, which aims to develop a new formulation of recombinant antitumor vaccines based on secretory granules with sustained release (SECRETVAC). Research supported by U1 (PPP) from Nanbiosis-ICTS and CIBER-BBN. The technology for artificial granule formation is patented by UAB and the Sant Pau Research Institute.
Eric Voltà-Durána,b,c, Julieta M Sáncheza,b,d,e, Eloi Parladéa,b,c, Ramon Manguesb,f, Antonio Villaverdea,b,c, Esther Vázqueza,b,c, Ugutz Unzuetab,c,f
aInstitut de Biotecnologia i de Biomedicina (IBB)
Universitat Autònoma de Barcelona
bCentro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina
Instituto de Salud Carlos III
cDepartment of Genetics and Microbiology
Universitat Autònoma de Barcelona
dDepartment of Chemistry
Universidad Nacional de Córdoba
eInstituto de Investigaciones Biológicas y Tecnológicas (IIByT)
Universidad Nacional de Córdoba
fInstitut de Recerca Sant Pau (IR SANT PAU)
Hospital de la Santa Creu i Sant Pau
References
Sanchez, J. M.; Voltà-Durán, E.; Parladé, E.; Mangues, R.; Villaverde, A.; Vázquez, E. & Unzueta, U. (2025). Surpassing protein specificity in biomimetics of bacterial amyloids. International journal of biological macromolecules, 296, 139635. https://doi.org/10.1016/j.ijbiomac.2025.139635

