Design of new compounds of Cu (II) with carboxylate ligands and pyridines
Supramolecular chemistry is an emergent research area that provides new complexes. These systems are composed of two or more assembled molecular units, which are connected by covalent bonds or weak intermolecular forces (hydrogen bond, van der Waals forces, and electrostatic effects, among others).
The synthesis and characterization of crystalline materials build up covalent bonds has gained interest in recent years, in particular the assembly of metal-carboxylates. These kind of compounds play an important role in synthetic chemistry for the design of porous materials with different metals as Cu(II) and Zn(II).
Recently, our research team has been studying the role of carboxylic acids and different amines in the structural arrangement.
In this work, we aimed to design news compounds of Cu(II) with carboxylate ligands (XCCO-) and pyridine/pyrazole ligands. The result obtained indicated the importance of the initial compound in the nuclearity of final compound (mononuclear, binuclear). We have synthesized and characterized six compounds; four are monomers and two dimers. All compounds were obtained from same initial compound. These six compounds were characterized by analytical, spectroscopic and single crystal X-ray diffraction.
This study allows to know the molecular and extended structures, obtaining 1D and 2D supramolecular networks. One of the monomers obtained shows in their structure three methanol and one occluded water molecules. The solvent accessible volume of the channels is a 6.2% of the cell volume. Under air exposure, the guest solvent is lost. Unfortunately, these changes seem to provoke the collapse of the pores, and the material does not absorb any significant amount of nitrogen.
Finally, for all these compounds, the UV-Vis spectra has been recorded, analyzed and compared with the spectra of other compounds described in the literature.
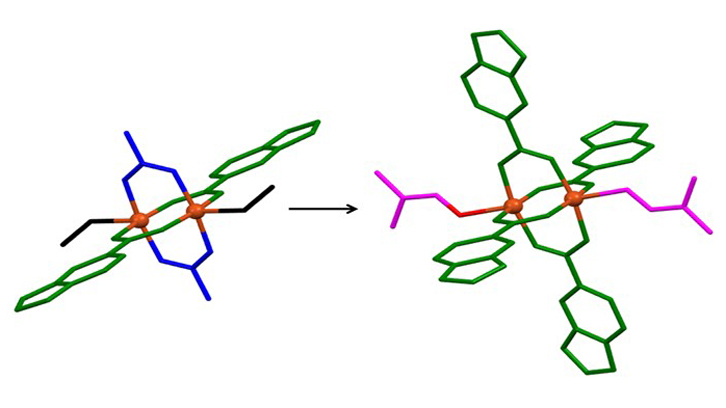
Figure 1. a) View of the one-dimensional ordering of one compound. b) Molecular structures of two compounds.
Department of Chemistry
Universitat Autònoma de Barcelona
References
Article de referència
Francisco Sánchez-Férez, Miguel Guerrero, José A. Ayllón, Teresa Calvet, Mercè Font-Bardía, José Giner Planas, Josefina Pons. (2019). Reactivity of homoleptic and heteroleptic core paddle-wheel Cu(II) compounds. Inorganica Chimica Acta, 487, 295-306. DOI: 10.1016/j.ica.2018.12.025.
Referències complementàries
- J. M. Lehn, Chem. Soc. Rev. 36 (2007) 151-160
- B. Olenyuk, M. D. Levin, J. A. Witeford, J. E. Shield, P.J. Stang, J. Am. Chem. Soc. 121 (1999) 10434-10435.
- J. Soldevila-Sanmartín, J. A. Ayllón, T. Clavet, M. Font-Bardía, C. Domingo, J. Pons, Inorg. Chem. Commun. 71 (2016) 90-93.
- M. Guerrero, S. Vázquez, J. A. Ayllón, T. Calvet, M. Font-Bardía, J. Pons, ChemistrySelect 2 (2017) 632-639.
- K. Hassanein, O. Castillo, C. J. Gómez-García, F. Zamora, P. Amo-Ochoa, Cryst. Growth Des. 15 (2015) 5485-5494.

