Gold, silver and copper in chemistry today
The synthesis, characterisation and the study of the specific properties of a great variety of complexes composed by organic molecules called ligands connected to metals have focused interesting studies in many laboratories for its potential applicability in a large quantity of fields. One of the extended lines in progressively expansion is the family of the compounds that contain N-pyrazole ligands.
In the last thirteen years, our group has realised great efforts to synthesise and characterise compounds that contain simple and hybrid N-pyrazole ligands. Hybrid ligands are composed by and N-pyrazole group and another functional group (with different electronic and steric properties in comparison with N-pyrazole group) capable to connect to the metallic centre. One of our research lines is the synthesis and characterisation of N-pyrazole, P-phosphine ligands. In particular, previously we synthesised the ligand (3,5-dimethyl-1H-pyrazol-1-yl)ethyldiphenylphosphine (L) hybrid ligand against Cu(I), Ag(I) and Au(I), and we have studied the reactivity against Rh(I) and Ru(II).
Metals of Group 11 are extensively investigated for their presence in biological systems and for their importance in the medical science. Also, the literature presents studies of Cu(I)/Cu(II) for its potential applicability in catalytic systems, silver for its historical photographic applications, and finally of gold for its organometallic applications.
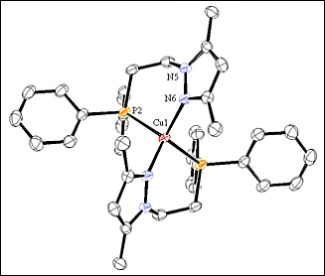
In this paper, we present the synthesis and characterisation of complexes Cu(I), Ag(I) and Au(I) with the ligand (3,5-dimethyl-1H-pyrazol-1-yl)ethyldiphenylphosphine (L), and we were obtained the compounds [Cu(L)2](PF6), [Ag(L)]2(PF6)2 and [AuCl(L)]2. These three compounds were characterised by elemental analyses, mass spectrometry, conductivity measurements, IR and NMR spectroscopies and single crystal X-ray diffraction. The structures of Cu(I) and Ag(I) compounds were confirmed by single crystal X-ray diffraction. With the information compiled in the characterisation of Ag(I) and Au(I), we observed a lot of similarities that permit to do a serious purpose of the structures of Au(I) in solid and solution. In this case, the tendency of the L ligand to coordinate to the metallic centre by the two active sites, and the presence of two chlorine atoms forming transannular double-bridging indicate the complex of Au(I) is a dimer.
Diagram of the cation [Ag(L)]22+ (L = 3,5-dimetil-1h-pirazol-1-il)etildifenil fosfina.
References
"Exploring the reactivity of an N-pyrazole, P-phosphine hybrid ligand with Cu(I), Ag(I) and Au(I) precursors". Sergio Muñoz, Josefina Pons, Josep Ros, Colin A. Kilner, Malcolm A. Halcrow. Journal of Organometallic Chemistry. 696;14,2011, pp2736-2741. doi:10.1016/j.jorganchem.2011.04.019

