A new study shows that rabbits are not resistant to prion infection
The research, coordinated by the Centre for Cooperative Research in Biosciences CIC bioGUNE has demonstrated in vivo that rabbits are not resistant to spongiform encephalopathies, neurodegenerative diseases caused by prions, although they appear to show a higher resistance than other mammals. The study, led by Dr. Joaquín Castilla, was held at the biosafety level 3 premises of IRTA-CReSA under the direction of Dr. Enric Vidal and with the collaboration of Dr. Belen Pintado from CNB. The project was funded by the MINECO (AGL2008-05296-C02). The study lasted four years and has been recently published in the journal PLoS Pathogens.
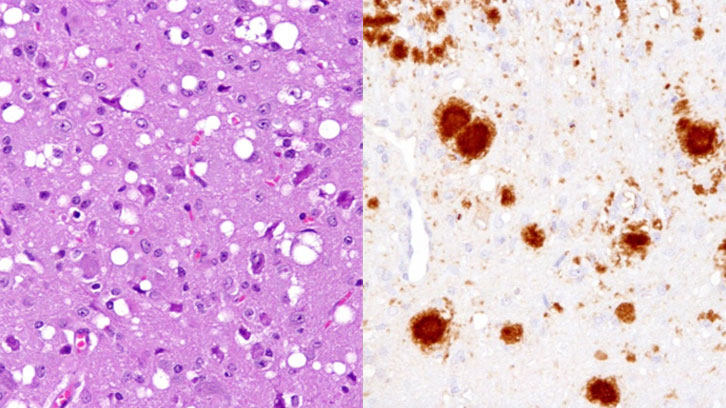
Prions are the infectious and abnormal version of the PrP protein which, in its benign form, is found in neurons of all mammals. When a prion –a word derived from “infectious protein particle” – is introduced into a healthy organism acts on the normal form of the protein, causing its misfolding and becoming a new prion. The accumulation of these abnormal and infectious proteins trigger neuronal death and cause the so called spongiform encephalopathies, neurodegenerative diseases that still have no cure or treatment. The spongy aspect under the microscope of the brains of sick animals originated the name of this group of diseases. One of the best known is the one that affects cattle, popularly known as the mad cow disease.
To conduct the research a line of transgenic mice was designed at the National Center for Biotechnology CNB-CSIC, in which the gene encoding for the mouse PrP protein was switched by the one corresponding to the rabbit PrP protein. “It is a smaller model than leporidae, but represents real rabbits very well and because of its cheaper cost allows us to conduct research with a much more significant number of individuals”.
Transgenic mice were then inoculated at CReSA’s biocontainment level 3 unit with real circulating prions from sheep, cow, mouse and deer. The researchers found that the animals developed a spongiform encephalopathy with the isolates obtained from cattle, mouse and sheep. However, there was no prion infection from the deer isolate. “For 40 years it was thought that the rabbit prion protein could not be misfolded. But this it is not the case, and this is the first fact we have proven. There is nothing in the rabbit protein that makes it resistant to misfolding”.
In 2012, the team led by Castilla laid the foundation stone to confirm prion infection in rabbits using a synthetic prion designed in the laboratory, but the current study is the in vivo verification with actual prions that were obtained from livestock species. “These findings should be taken into account when feeding the rabbits, especially if feeds containing animal protein are used”.
Different studies, identical conclusions
In addition, results are published in the same issue of a study, carried out by scientists from the Institute of Agronomic Research (INRA, France), who have directly worked with transgenic rabbits in which the gene encoding for ovine PrP was introduced. The researchers then inoculated them with sheep prion isolates, between six and eight months later, these rabbits were sick with a spongiform encephalopathy. Thus, this study also concludes that there is nothing in the rabbit’s genome that makes rabbits resistant to prion diseases.
Why is it so hard to make the rabbits go mad?
For 4 decades it has believed that leporidae were resistant to prion infection, based on the lack of field cases of spongiform encephalopathies in rabbits. The most recent research has disproved that theory and so far has not been able to uncover the reasons why rabbits seem to be more resistant to prion diseases than other mammals. The absence of cases of rabbits with this disease may lie in the fact that leporidae may have not been exposed to animal protein feed derived from infected animals as happened with other species. Another key lies in the age of the animal. Spongiform encephalopathies generally have a long incubation and rabbits are slaughtered at an early age.
Centre de Recerca en Sanitat Animal (CReSA)
IRTA
References
Vidal, E.; Fernández-Borges, N.; Pintado, B.; Eraña, H.; Ordóñez, M.; Márquez, M. et al. Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates. PLoS Pathogens. 2015, vol. 11, num. 8, e1004977. doi: 10.1371/journal.ppat.1004977.


